In the preparation of human cells used for regenerative medicine, it is essential that the product is sterile. This is because the product is directly administered or transplanted into the body. As such, cell processing centers (CPC: cell processing center) are used to process cells in a sterile environment to ensure the necessary product quality.
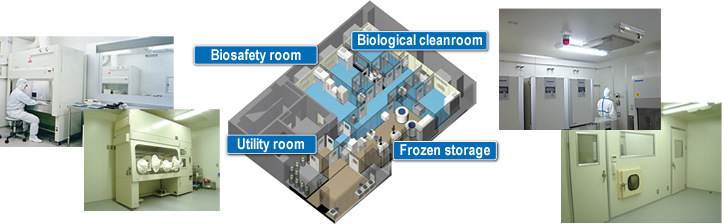
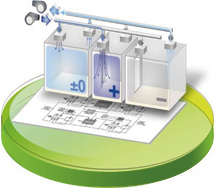
In the design stage, room pressure fluctuation attributable to external air pressure and the opening and closing of doors is predicted with high accuracy, achieving a facility capable of stable room pressure control.

An information system incorporating a server that reduces human error and saves labor in factors such as manufacturing planning, work instructions, work records, quality control, and form creation.

A system that monitors and records temperature and humidity, differential pressure between rooms, particles, and so on, in real time. It stores operating data of equipment, and alerts operators in the event of an abnormality.